Covalent Bond Weak Or Strong

A covalent bond is a chemical bond that involves the sharing of electrons to course electron pairs betwixt atoms. These electron pairs are known every bit shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.[ane] For many molecules, the sharing of electrons allows each cantlet to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemical science, covalent bonding is much more mutual than ionic bonding.
Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and 3-eye iv-electron bonds.[two] [iii] The term covalent bond dates from 1939.[4] The prefix co- means jointly, associated in activity, partnered to a lesser degree, etc.; thus a "co-valent bond", in essence, ways that the atoms share "valence", such as is discussed in valence bond theory.
In the molecule H
2 , the hydrogen atoms share the two electrons via covalent bonding.[5] Covalency is greatest between atoms of like electronegativities. Thus, covalent bonding does non necessarily require that the two atoms be of the same elements, only that they exist of comparable electronegativity. Covalent bonding that entails the sharing of electrons over more than two atoms is said to be delocalized.
History [edit]
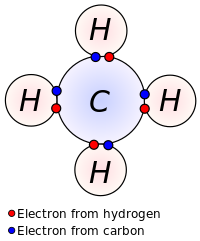
Early concepts in covalent bonding arose from this kind of image of the molecule of methane. Covalent bonding is implied in the Lewis construction by indicating electrons shared between atoms.
The term covalence in regard to bonding was first used in 1919 by Irving Langmuir in a Periodical of the American Chemical Society article entitled "The Arrangement of Electrons in Atoms and Molecules". Langmuir wrote that "we shall announce by the term covalence the number of pairs of electrons that a given atom shares with its neighbors."[six]
The idea of covalent bonding tin be traced several years before 1919 to Gilbert Due north. Lewis, who in 1916 described the sharing of electron pairs between atoms.[7] He introduced the Lewis notation or electron dot note or Lewis dot structure, in which valence electrons (those in the outer shell) are represented as dots around the atomic symbols. Pairs of electrons located between atoms represent covalent bonds. Multiple pairs represent multiple bonds, such every bit double bonds and triple bonds. An culling form of representation, not shown hither, has bail-forming electron pairs represented as solid lines.[viii]
Lewis proposed that an atom forms enough covalent bonds to grade a total (or closed) outer electron shell. In the diagram of methane shown hither, the carbon atom has a valence of 4 and is, therefore, surrounded by viii electrons (the octet rule), iv from the carbon itself and four from the hydrogens bonded to it. Each hydrogen has a valence of i and is surrounded by two electrons (a duet dominion) – its own one electron plus 1 from the carbon. The numbers of electrons correspond to full shells in the breakthrough theory of the cantlet; the outer trounce of a carbon cantlet is the northward = 2 shell, which can hold 8 electrons, whereas the outer (and merely) shell of a hydrogen cantlet is the due north = 1 shell, which tin concord only two.[9]
While the thought of shared electron pairs provides an effective qualitative film of covalent bonding, quantum mechanics is needed to understand the nature of these bonds and predict the structures and backdrop of simple molecules. Walter Heitler and Fritz London are credited with the first successful quantum mechanical explanation of a chemical bond (molecular hydrogen) in 1927.[ten] Their work was based on the valence bond model, which assumes that a chemical bond is formed when at that place is good overlap betwixt the atomic orbitals of participating atoms.
Types of covalent bonds [edit]
Atomic orbitals (except for south orbitals) have specific directional properties leading to different types of covalent bonds. Sigma (σ) bonds are the strongest covalent bonds and are due to head-on overlapping of orbitals on two different atoms. A unmarried bond is commonly a σ bond. Pi (π) bonds are weaker and are due to lateral overlap between p (or d) orbitals. A double bond between ii given atoms consists of ane σ and i π bond, and a triple bond is one σ and 2 π bonds.[viii]
Covalent bonds are also affected by the electronegativity of the connected atoms which determines the chemical polarity of the bail. Two atoms with equal electronegativity volition brand nonpolar covalent bonds such every bit H–H. An unequal relationship creates a polar covalent bail such as with H−Cl. Nevertheless polarity besides requires geometric asymmetry, or else dipoles may cancel out, resulting in a not-polar molecule.[viii]
Covalent structures [edit]
In that location are several types of structures for covalent substances, including individual molecules, molecular structures, macromolecular structures and behemothic covalent structures. Individual molecules have strong bonds that hold the atoms together, but generally, at that place are negligible forces of attraction between molecules. Such covalent substances are usually gases, for instance, HCl, SO2, CO2, and CH4. In molecular structures, there are weak forces of allure. Such covalent substances are low-boiling-temperature liquids (such as ethanol), and low-melting-temperature solids (such as iodine and solid CO2). Macromolecular structures take big numbers of atoms linked by covalent bonds in bondage, including synthetic polymers such every bit polyethylene and nylon, and biopolymers such equally proteins and starch. Network covalent structures (or behemothic covalent structures) contain big numbers of atoms linked in sheets (such as graphite), or 3-dimensional structures (such as diamond and quartz). These substances have loftier melting and boiling points, are frequently brittle, and tend to have high electric resistivity. Elements that have high electronegativity, and the ability to form three or iv electron pair bonds, frequently form such large macromolecular structures.[11]
One- and three-electron bonds [edit]

Bonds with ane or three electrons can be found in radical species, which have an odd number of electrons. The simplest example of a 1-electron bond is found in the dihydrogen cation, H +
ii . One-electron bonds frequently have about half the bond free energy of a two-electron bail, and are therefore chosen "one-half bonds". Yet, there are exceptions: in the case of dilithium, the bail is actually stronger for the 1-electron Li +
ii than for the 2-electron Litwo. This exception tin be explained in terms of hybridization and inner-trounce effects.[12]
The simplest example of 3-electron bonding can be found in the helium dimer cation, He +
2 . It is considered a "half bond" considering it consists of only one shared electron (rather than two);[13] in molecular orbital terms, the third electron is in an anti-bonding orbital which cancels out half of the bond formed by the other 2 electrons. Another example of a molecule containing a 3-electron bond, in addition to 2 2-electron bonds, is nitric oxide, NO. The oxygen molecule, O2 can also be regarded as having two 3-electron bonds and one ii-electron bond, which accounts for its paramagnetism and its formal bail order of 2.[fourteen] Chlorine dioxide and its heavier analogues bromine dioxide and iodine dioxide as well contain three-electron bonds.
Molecules with odd-electron bonds are usually highly reactive. These types of bond are only stable between atoms with like electronegativities.[14]

Nitric oxide
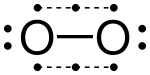
Dioxygen
Resonance [edit]
There are situations whereby a unmarried Lewis construction is insufficient to explicate the electron configuration in a molecule and its resulting experimentally-determined properties, hence a superposition of structures is needed. The same two atoms in such molecules can be bonded differently in different Lewis structures (a single bond in one, a double bail in some other, or even none at all), resulting in a non-integer bond social club. The nitrate ion is ane such example with iii equivalent structures. The bail between the nitrogen and each oxygen is a double bond in one structure and a single bond in the other two, so that the average bond order for each North–O interaction is 2 + 1 + 1 / 3 = 4 / three .[8]

Aromaticity [edit]
In organic chemistry, when a molecule with a planar band obeys Hückel'due south rule, where the number of π electrons fit the formula 4n + 2 (where n is an integer), information technology attains actress stability and symmetry. In benzene, the prototypical aromatic compound, there are half dozen π bonding electrons (due north = one, fourdue north + ii = 6). These occupy three delocalized π molecular orbitals (molecular orbital theory) or course conjugate π bonds in ii resonance structures that linearly combine (valence bond theory), creating a regular hexagon exhibiting a greater stabilization than the hypothetical i,3,5-cyclohexatriene.[9]
In the case of heterocyclic aromatics and substituted benzenes, the electronegativity differences between different parts of the ring may dominate the chemic beliefs of aromatic ring bonds, which otherwise are equivalent.[9]
Hypervalence [edit]
Certain molecules such as xenon difluoride and sulfur hexafluoride have higher co-ordination numbers than would be possible due to strictly covalent bonding according to the octet rule. This is explained by the three-center iv-electron bond ("3c–4e") model which interprets the molecular wavefunction in terms of not-bonding highest occupied molecular orbitals in molecular orbital theory and resonance of sigma bonds in valence bond theory.[15]
Electron deficiency [edit]
In iii-center two-electron bonds ("3c–2e") iii atoms share two electrons in bonding. This blazon of bonding occurs in boron hydrides such equally diborane (B2H6), which are often described equally electron deficient because at that place are not enough valence electrons to grade localized (2-eye 2-electron) bonds joining all the atoms. Nonetheless the more modern description using 3c–2e bonds does provide plenty bonding orbitals to connect all the atoms, and then that the molecules tin instead be classified as electron-precise.
Each such bond (2 per molecule in diborane) contains a pair of electrons which connect the boron atoms to each other in a banana shape, with a proton (the nucleus of a hydrogen cantlet) in the centre of the bond, sharing electrons with both boron atoms. In sure cluster compounds, so-chosen 4-center two-electron bonds also accept been postulated.[16]
Quantum mechanical description [edit]
Subsequently the development of quantum mechanics, two basic theories were proposed to provide a quantum description of chemical bonding: valence bail (VB) theory and molecular orbital (MO) theory. A more than recent breakthrough description[17] is given in terms of atomic contributions to the electronic density of states.
Comparing of VB and MO theories [edit]
The two theories represent two means to build upward the electron configuration of the molecule.[eighteen] For valence bail theory, the atomic hybrid orbitals are filled with electrons first to produce a fully bonded valence configuration, followed past performing a linear combination of contributing structures (resonance) if there are several of them. In contrast, for molecular orbital theory a linear combination of atomic orbitals is performed offset, followed by filling of the resulting molecular orbitals with electrons.[viii]
The two approaches are regarded as complementary, and each provides its own insights into the problem of chemic bonding. Every bit valence bond theory builds the molecular wavefunction out of localized bonds, information technology is more suited for the calculation of bond energies and the understanding of reaction mechanisms. As molecular orbital theory builds the molecular wavefunction out of delocalized orbitals, it is more suited for the calculation of ionization energies and the understanding of spectral absorption bands.[xix]
At the qualitative level, both theories comprise incorrect predictions. Uncomplicated (Heitler–London) valence bond theory correctly predicts the dissociation of homonuclear diatomic molecules into separate atoms, while elementary (Hartree–Fock) molecular orbital theory incorrectly predicts dissociation into a mixture of atoms and ions. On the other hand, simple molecular orbital theory correctly predicts Hückel's rule of aromaticity, while unproblematic valence bond theory incorrectly predicts that cyclobutadiene has larger resonance energy than benzene.[20]
Although the wavefunctions generated by both theories at the qualitative level do not agree and exercise non match the stabilization energy by experiment, they can be corrected by configuration interaction.[eighteen] This is done past combining the valence bail covalent function with the functions describing all possible ionic structures or by combining the molecular orbital ground land function with the functions describing all possible excited states using unoccupied orbitals. It can so be seen that the uncomplicated molecular orbital approach overestimates the weight of the ionic structures while the elementary valence bond arroyo neglects them. This can also be described as saying that the unproblematic molecular orbital approach neglects electron correlation while the simple valence bond approach overestimates it.[18]
Mod calculations in quantum chemistry normally start from (but ultimately go far beyond) a molecular orbital rather than a valence bond approach, not because of any intrinsic superiority in the sometime but rather because the MO arroyo is more than readily adapted to numerical computations. Molecular orbitals are orthogonal, which significantly increases the feasibility and speed of computer calculations compared to nonorthogonal valence bond orbitals.
Covalency from atomic contribution to the electronic density of states [edit]
In COOP,[21] COHP[22] and BCOOP,[23] evaluation of bond covalency is dependent on the basis ready. To overcome this consequence, an alternative conception of the bail covalency can be provided in this way.
The center mass of an atomic orbital with quantum numbers for atom A is defined as
where is the contribution of the atomic orbital of the cantlet A to the total electronic density of states of the solid
where the outer sum runs over all atoms A of the unit cell. The energy window is chosen in such a way that information technology encompasses all of the relevant bands participating in the bond. If the range to select is unclear, it can be identified in practice past examining the molecular orbitals that describe the electron density along with the considered bail.
The relative position of the center mass of levels of cantlet A with respect to the center mass of levels of atom B is given every bit
where the contributions of the magnetic and spin quantum numbers are summed. According to this definition, the relative position of the A levels with respect to the B levels is
where, for simplicity, we may omit the dependence from the principal quantum number in the notation referring to
In this formalism, the greater the value of the higher the overlap of the selected atomic bands, and thus the electron density described past those orbitals gives a more covalent A−B bond. The quantity is denoted as the covalency of the A−B bail, which is specified in the same units of the energy .
Analogous effect in nuclear systems [edit]
An analogous effect to covalent binding is believed to occur in some nuclear systems, with the difference that the shared fermions are quarks rather than electrons.[24] High energy proton-proton scattering cross-department indicates that quark interchange of either u or d quarks is the dominant procedure of the nuclear forcefulness at short distance. In particular, information technology dominates over the Yukawa interaction where a meson is exchanged.[25] Therefore, covalent binding by quark interchange is expected to be the dominating mechanism of nuclear binding at small distance when the spring hadrons take covalence quarks in common.[26]
See too [edit]
- Bonding in solids
- Bail order
- Coordinate covalent bail, also known every bit a dipolar bail or a dative covalent bond
- Covalent bond classification (or LXZ notation)
- Covalent radius
- Disulfide bond
- Hybridization
- Hydrogen bond
- Ionic bond
- Linear combination of diminutive orbitals
- Metallic bonding
- Noncovalent bonding
- Resonance (chemistry)
References [edit]
- ^ Whitten, Kenneth Due west.; Gailey, Kenneth D.; Davis, Raymond East. (1992). "seven-iii Germination of covalent bonds". Full general Chemistry (4th ed.). Saunders College Publishing. p. 264. ISBN0-03-072373-6.
- ^ March, Jerry (1992). Advanced Organic Chemical science: Reactions, Mechanisms, and Construction . John Wiley & Sons. ISBN0-471-60180-2.
- ^ Gary 50. Miessler; Donald Arthur Tarr (2004). Inorganic Chemical science . Prentice Hall. ISBN0-13-035471-vi.
- ^ Merriam-Webster – Collegiate Lexicon (2000).
- ^ "Chemical Bonds". Hyperphysics.phy-astr.gsu.edu. Retrieved 2013-06-09 .
- ^ Langmuir, Irving (1919-06-01). "The System of Electrons in Atoms and Molecules". Periodical of the American Chemical Society. 41 (6): 868–934. doi:10.1021/ja02227a002.
- ^ Lewis, Gilbert N. (1916-04-01). "The atom and the molecule". Journal of the American Chemic Lodge. 38 (4): 762–785. doi:10.1021/ja02261a002. S2CID 95865413.
- ^ a b c d e McMurry, John (2016). Chemical science (7 ed.). Pearson. ISBN978-0-321-94317-0.
- ^ a b c Bruice, Paula (2016). Organic Chemistry (viii ed.). Pearson. ISBN978-0-13-404228-ii.
- ^ Heitler, W.; London, F. (1927). "Wechselwirkung neutraler Atome und homöopolare Bindung nach der Quantenmechanik" [Interaction of neutral atoms and homeopolar bonds according to breakthrough mechanics]. Zeitschrift für Physik. 44 (6–7): 455–472. Bibcode:1927ZPhy...44..455H. doi:ten.1007/bf01397394. S2CID 119739102. English translation in Hettema, H. (2000). Breakthrough Chemistry: Archetype Scientific Papers. Globe Scientific. p. 140. ISBN978-981-02-2771-v . Retrieved 2012-02-05 .
- ^ Stranks, D. R.; Heffernan, Yard. L.; Lee Dow, Chiliad. C.; McTigue, P. T.; Withers, Grand. R. A. (1970). Chemical science: A structural view. Carlton, Vic.: Melbourne Academy Press. p. 184. ISBN0-522-83988-half dozen.
- ^ Weinhold, F.; Landis, C. (2005). Valency and Bonding. Cambridge. pp. 96–100. ISBN0-521-83128-eight.
- ^ Harcourt, Richard D., ed. (2015). "Chapter two: Pauling "3-Electron Bonds", 4-Electron 3-Centre Bonding, and the Demand for an "Increased-Valence" Theory". Bonding in Electron-Rich Molecules: Qualitative Valence-Bond Approach via Increased-Valence Structures. Springer. ISBN9783319166766.
- ^ a b Pauling, L. (1960). The Nature of the Chemical Bond . Cornell University Press. pp. 340–354.
- ^ Weinhold, F.; Landis, C. (2005). Valency and Bonding. Cambridge Academy Press. pp. 275–306. ISBN0521831288.
- ^ Hofmann, K.; Prosenc, Thousand. H.; Albert, B. R. (2007). "A new 4c–2e bail in B
6 H −
seven ". Chemical Communications. 2007 (29): 3097–3099. doi:x.1039/b704944g. PMID 17639154. - ^ Cammarata, Antonio; Rondinelli, James M. (21 September 2014). "Covalent dependence of octahedral rotations in orthorhombic perovskite oxides". Journal of Chemical Physics. 141 (xi): 114704. Bibcode:2014JChPh.141k4704C. doi:x.1063/1.4895967. PMID 25240365.
- ^ a b c Atkins, P. Due west. (1974). Quanta: A Handbook of Concepts. Oxford Academy Printing. pp. 147–148. ISBN978-0-19-855493-6.
- ^ James D. Ingle Jr. and Stanley R. Hunker, Spectrochemical Analysis, Prentice Hall, 1988, ISBN 0-13-826876-2
- ^ Anslyn, Eric V. (2006). Modernistic Physical Organic Chemistry. University Science Books. ISBN978-1-891389-31-iii.
- ^ Hughbanks, Timothy; Hoffmann, Roald (2002-05-01). "Chains of trans-edge-sharing molybdenum octahedra: metal-metal bonding in extended systems". Journal of the American Chemical Society. 105 (eleven): 3528–3537. doi:10.1021/ja00349a027.
- ^ Dronskowski, Richard; Bloechl, Peter E. (2002-05-01). "Crystal orbital Hamilton populations (COHP): free energy-resolved visualization of chemical bonding in solids based on density-functional calculations". The Journal of Physical Chemistry. 97 (33): 8617–8624. doi:10.1021/j100135a014.
- ^ Grechnev, Alexei; Ahuja, Rajeev; Eriksson, Olle (2003-01-01). "Balanced crystal orbital overlap population—a tool for analysing chemical bonds in solids". Journal of Physics: Condensed Matter. 15 (45): 7751. Bibcode:2003JPCM...15.7751G. doi:x.1088/0953-8984/xv/45/014. ISSN 0953-8984. S2CID 250757642.
- ^ Brodsky, S. J. (2017). "Novel Features of Nuclear Chromodynamics". The European Physical Journal A. 53 (3): 48. Bibcode:2017EPJA...53...48B. doi:10.1140/epja/i2017-12234-5. OSTI 1341388. S2CID 126305939.
- ^ Brodsky, S. J.; Mueller, A. H. (1988). "Using Nuclei to Probe Hadronization in QCD". Physics Messages B. 206 (4): 685. Bibcode:1988PhLB..206..685B. doi:10.1016/0370-2693(88)90719-eight.
- ^ Bashkanova, 1000.; Brodsky, S. J.; Cloudless, H. (2013). "Novel Six-Quark Hidden-Colour Dibaryon States in QCD". Physics Messages B. 727 (4–5): 438. arXiv:1308.6404. Bibcode:2013PhLB..727..438B. doi:10.1016/j.physletb.2013.x.059. S2CID 30153514.
Sources [edit]
- "Covalent bonding – Single bonds". chemguide. 2000. Retrieved 2012-02-05 .
- "Electron Sharing and Covalent Bonds". Department of Chemistry University of Oxford. Retrieved 2012-02-05 .
- "Chemical Bonds". Department of Physics and Astronomy, Georgia Land University. Retrieved 2012-02-05 .
External links [edit]
- Covalent Bonds and Molecular Structure
- Structure and Bonding in Chemistry—Covalent Bonds
Covalent Bond Weak Or Strong,
Source: https://en.wikipedia.org/wiki/Covalent_bond
Posted by: zaratenowbod.blogspot.com












![{\displaystyle [E_{0},E_{1}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/972145e0fa9d0c7fe3d06950100d008bb94d3729)











0 Response to "Covalent Bond Weak Or Strong"
Post a Comment